usp class vi compliant
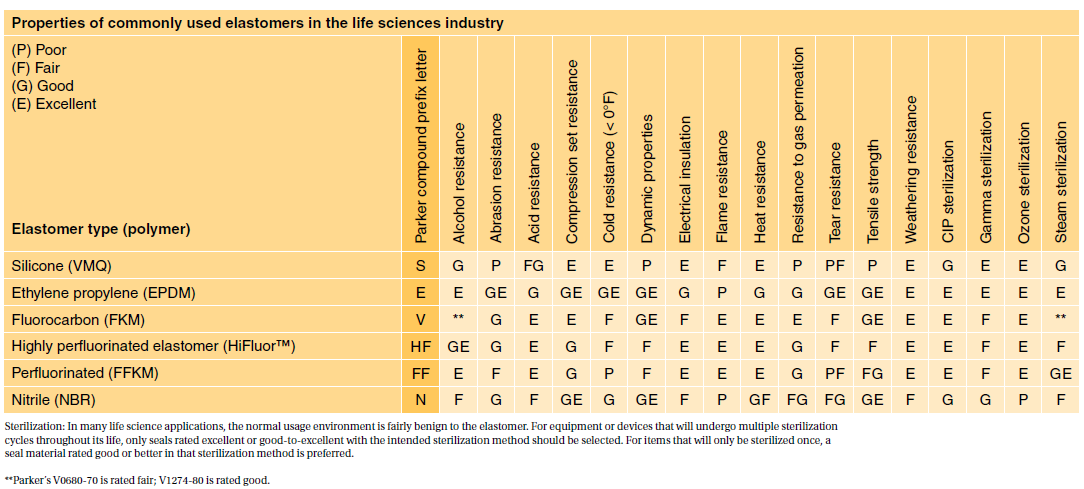
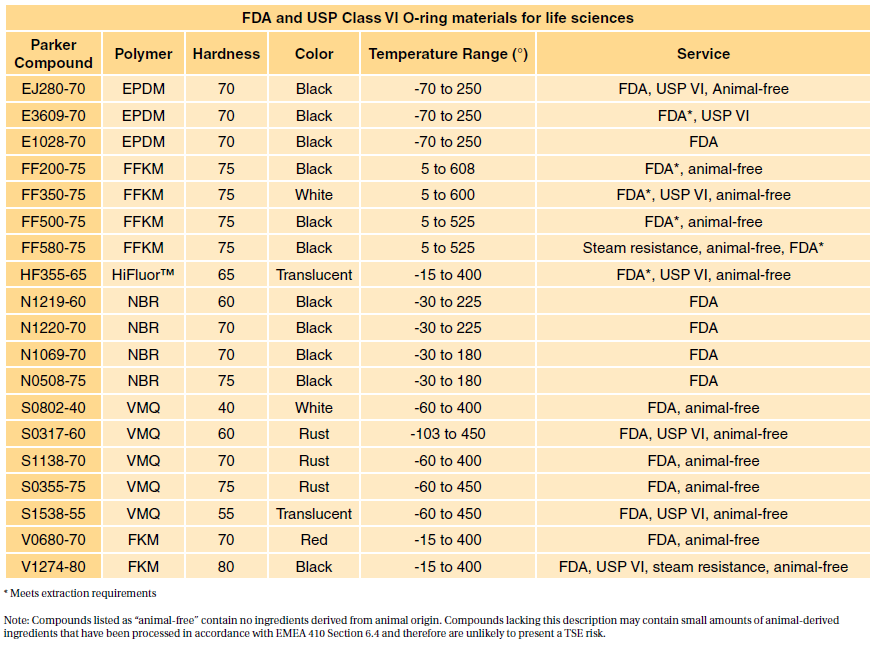
Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries. Sil 714002 USP class VI Silicone 1 70 Yes transl.

Cpc Usp Class Vi Compliant Polysulfone High Flow Quick Disconnect Couplings Cole Parmer
Class VI Test USP Project Number.

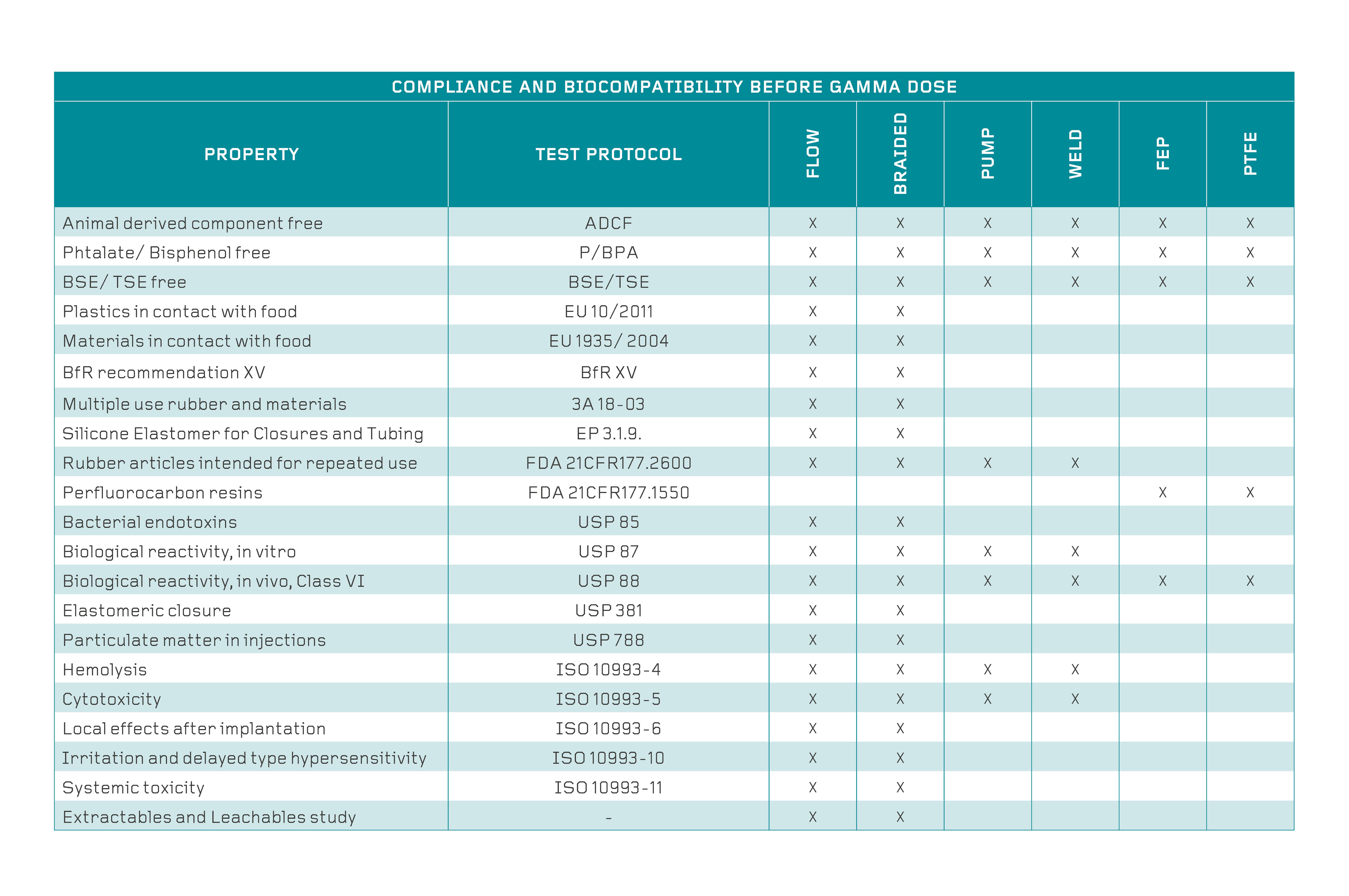
. USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. SIMONA PP-H USP Class VI sheet material is easy to clean and disinfect using most hospital grade cleaners and disinfectants. USP Class VI Certificate of Compliance.
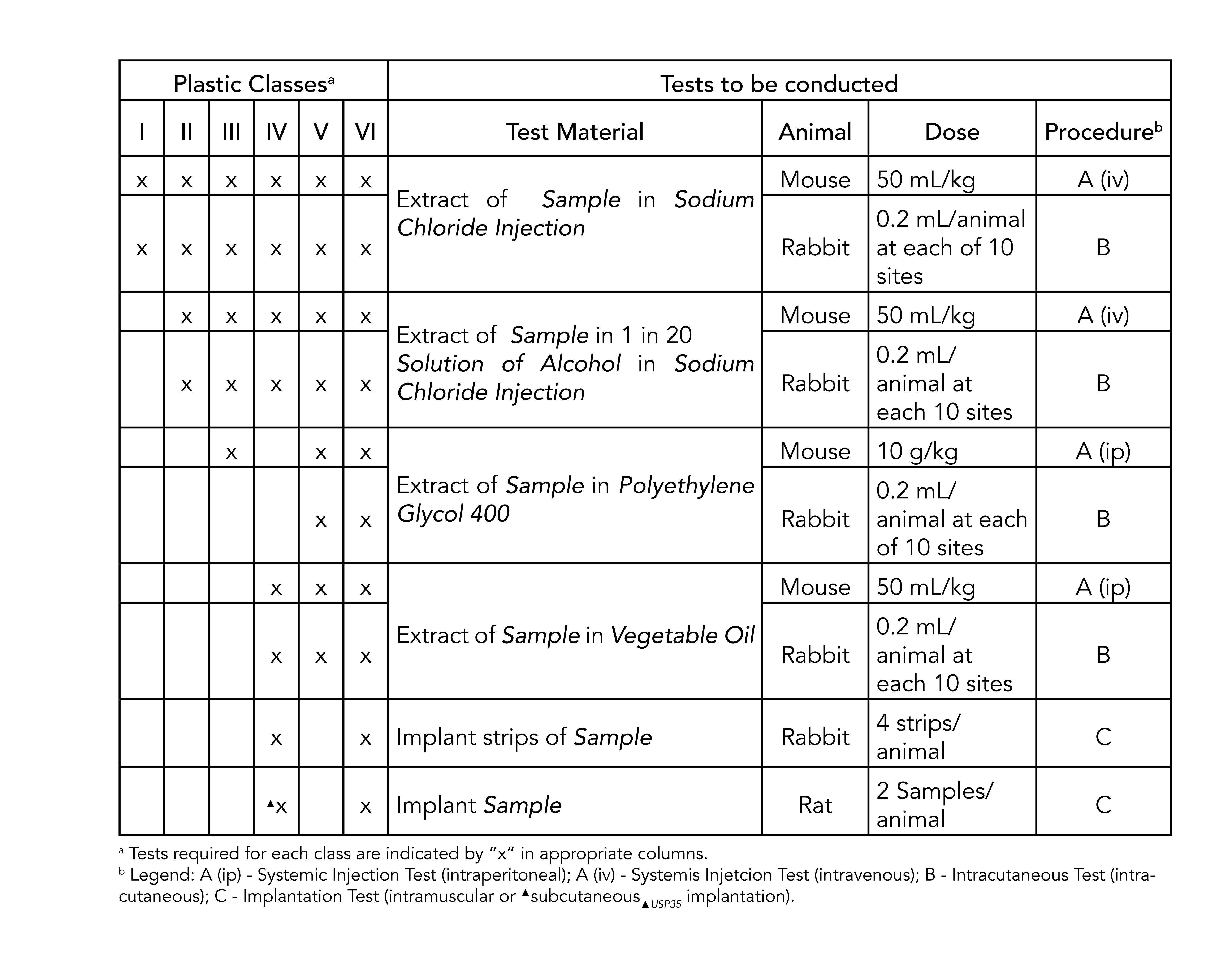
Plastics are classified into one of six classes each requiring different levels of testing. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. Food and Drug Administration FDA.
AFT Fluorotec can manufacture a wide range of components using our USP Class VI PTFE compliant material and are supplying customers worldwide to meet their requirements. Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. Suitability under USP Class VI is typically a base requirement for medical device manufacturers.
Tests of the provided material samples passed all requirements and have been approved for. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. I know that performing a USP Class VI test even for a 30 day period will still not perform to ISO10993-1 per General ProgramBluebook Memo G95-1 We are.
High quality USP Class VI compliant sheet material made from our own specially formulated compounds. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. The test article was.
Manufactures standard and custom size o-rings compliant to the US. Watershed 11122XC 70 EXPERIMENTAL DESIGN AND DOSAGE 71 Preparation of Test and Control Articles. Pawling Engineered Products Inc.
EPFEPerfluoroelastomer Seals Special RubberFabrications. Class VI requires the most extensive testing. Graco Company have been tested for compliance to USP Class VI 70C plastic.
There are six classes VI being the most rigorous. Table 1 shows our standard programme FDA compliant com-pounds which can be produced in a few days. Compliance to USP Class VI is often requested by end users.
Material is ordered by the linear inch and is most suited for die cutting parts. Sterile and diaphragm valves have USP Class VI PTFE material in them and sanitary pumps require Class VI O-Rings and sealing material. 157 Charles Colman Boulevard Pawling NY 12564.
USP does not regulate compliance or certification of plastics tested according to their published methods. Theres even a silicon carbide surface thats compliant and various release agents used in the manufacture of silicone plastics are compliant. USP Class Testing standards are determined by the United States.
711 Systemic and Intracutaneous Testing Preparation. Pharmacopoeia USP Class VI requirements. USP Class VI refers to a set of biocompatibility testing requirements from the US.
USP Class VI compliant Extrusions Cord. Sheet material is available in various thick-nesses and with a standard of 36 width. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment.
Specially formulated for long term sealing. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. EPDM red Silicone and VitonTM.
7111 The test article 60 cm2 was combined with 10 mL of vehicle at a ratio of 120 cm2 per 20 mL per USP guidelines. Specially formulated for long term sealing. RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials.
7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600. What is ADI-Free BSE-Free TSE-Free. United States Pharmacopeia USP 26 NF21 2003 Class VI.
Standards are published in the US Pharmocopeia and the National Formulary USP NF. Phone 845 855-1000 800 431-0101 Fax 845 855-1139. Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices.
When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot. There are plenty of silicone products and other medical grade plastics that are USP Class VI compliant. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.
But Dursan is one of the first CVD silicon coatings to achieve USP Class VI compliance. So here is a new one - a customer has requested us to conduct testing compliant to USP Class VI and ISO10993-1 compliant. Sil 714001 USP class VI Silicone 1 70 Yes transl.
Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. All these special grade products have passed this rigorous test. In addition SIMONA PP-H USP Class VI sheet delivers high chemical and corrosion resistance excellent surface.
Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US. Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. What is USP Class VI compliant.
IEGeek - 2006. ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for.
The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification. The FDA has adopted some the tests specified by USP for regulation of medical devices. USP Class VI materials are available in 70 durometer EPDM Silicone and Viton.
Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. Compounds made without animal-derived ingredients BSETSE concerns. Testing for compliance involves an assessment of the effects of the material and extractables on tissue.
USP Class VI compliant compounds are formulated with ingredients providing certifiable biocompatibility and low extractables for system toxicity and purity. Testing was performed by Pacific BioLabs on September 16 2015 in compliance with the standards published in the USP Biocompatibility Testing standards USP. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal

Usp Class Vi O Rings And Seals Eastern Seals Uk Ltd

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc
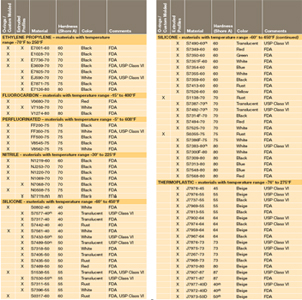
Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Usp Class Plastics Pacific Biolabs

What Is Usp Class Vi Testing Tbl Plastics

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Usp Class Vi Foster Corporation
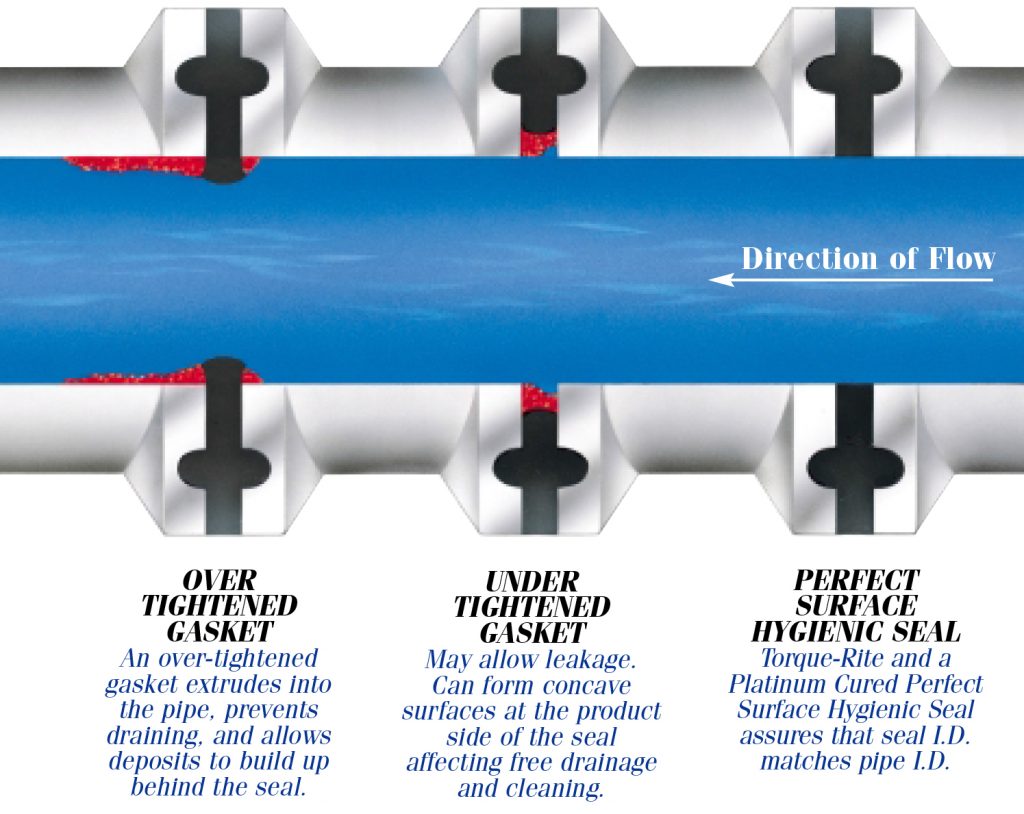
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa